Volatile Organic Compounds (VOCs)
Volatile Organic Compounds are compounds that have a high vapor pressure and low water solubility.
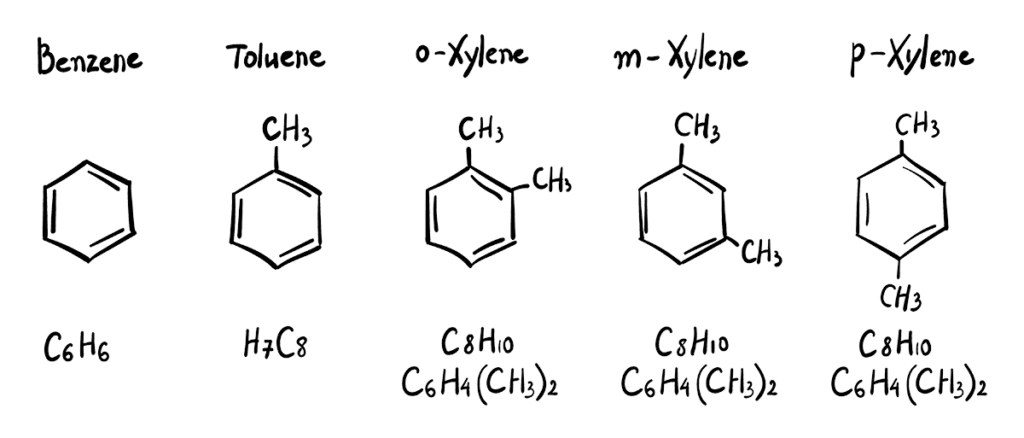
Examples of common VOCs: Benzene methyl group – benzene, toluene, o-Xylene, meta-xylene, and para-xylene.
Many VOCs are human-made chemicals that are used and produced in the manufacture of paints, pharmaceuticals, and refrigerants. VOCs typically are industrial solvents, such as trichloroethylene; fuel oxygenates, such as methyl tert-butyl ether (MTBE); or by-products produced by chlorination in water treatment, such as Trihalomethanes. VOCs are often components of petroleum fuels, hydraulic fluids, paint thinners, and dry cleaning agents. VOCs are common ground-water contaminants.
Volatile organic compounds (VOCs) are emitted as gases from certain solids or liquids. VOCs include a variety of chemicals, some of which may have short- and long-term adverse health effects. Concentrations of many VOCs are consistently higher indoors (up to ten times higher) than outdoors. VOCs are emitted by a wide array of products numbering in the thousands. Examples include: paints and lacquers, paint strippers, cleaning supplies, pesticides, building materials and furnishings, office equipment such as copiers and printers, correction fluids and carbonless copy paper, graphics and craft materials including glues and adhesives, permanent markers, and photographic solutions.
 Organic chemicals are widely used as ingredients in household products. Paints, varnishes, and wax all contain organic solvents, as do many cleaning, disinfecting, cosmetic, degreasing, and hobby products. Fuels are made up of organic chemicals. All of these products can release organic compounds while you are using them, and, to some degree, when they are stored.
Organic chemicals are widely used as ingredients in household products. Paints, varnishes, and wax all contain organic solvents, as do many cleaning, disinfecting, cosmetic, degreasing, and hobby products. Fuels are made up of organic chemicals. All of these products can release organic compounds while you are using them, and, to some degree, when they are stored.
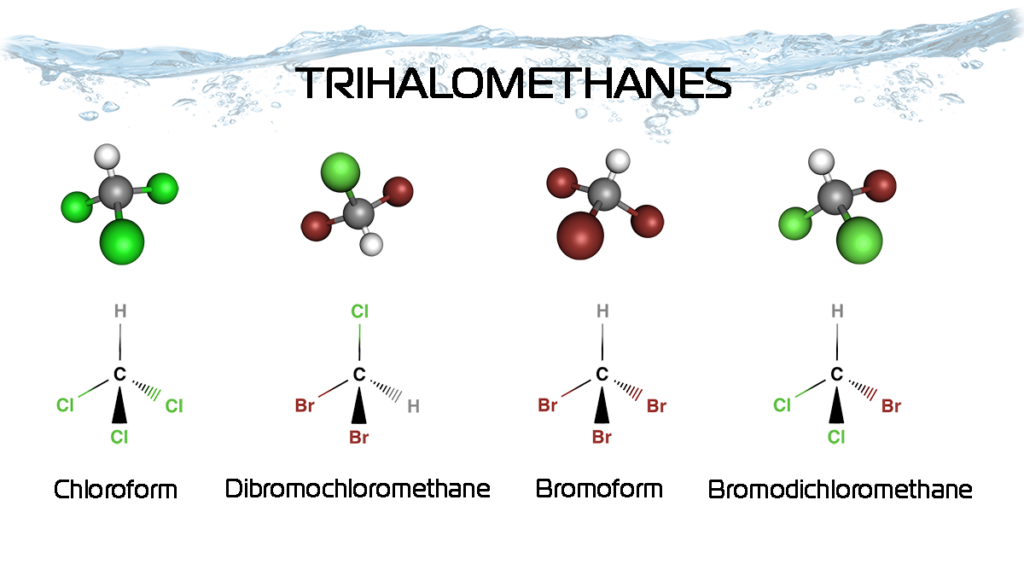
WHAT ARE TRIHALOMETHANES (THMs)?
Source: https://wellowner.org/resources/water-quality/contaminants/trihalomethanes/
Trihalomethanes are a group of four chemicals – chloroform, bromoform, bromodichloromethane, and dibromochloromethane.
These compounds are formed, along with other disinfection by-products, when chlorine, bromine, or other disinfectants used to control microbial contaminants in drinking water react with naturally occurring methane, often derived from other forms of organic and inorganic matter in water, usually broken up by chlorine or bromine used to disinfect water. THMs are part of a larger class of halogenated organic compounds, including others of water supply health concern such as halogenated acetic acids (HAA). THM are the most common halogenated organics.
How are they formed?
Chloroform—the trihalomethane often found in the highest concentration—is formed by a reaction of chlorine with certain compounds in water. Formation occurs during chlorination and can continue to occur as long as chlorine is available. The other trihalomethanes are formed by a reaction of bromine and iodine with the same certain compounds.
Depending on the characteristics of the water, the other three trihalomethanes may be formed at a higher concentration than chloroform.
Where do you find Trihalomethanes?
Trihalomethanes are much more prevalent in public water supplies because most use chlorination as a disinfection technology. However, while trihalomethanes are more common in the public water systems, they are a threat to any water supply that uses chlorine—including private water wells.
How dangerous are they?
High levels of trihalomethanes can be dangerous. In fact, in December 2000, the U.S. Environmental Protection Agency lowered the maximum allowable annual average level for large surface water public water systems from 100 parts per billion (ppb) to 80 ppb. The 80 ppb limit goes into effect for small surface water and all ground water systems in December 2003.
What are the signs of exposure and who is at risk?
Some studies have suggested a small increase in the risk of bladder and colorectal cancers. Other investigations have found that chlorination by-products may be linked to heart, lung, kidney, liver, and central nervous system damage.
Of the different trihalomethanes, dibromochloromethane has been most closely associated with cancer, followed in order by bromoform, chloroform, and bromodichloromethane.
Pregnant women appear to be at the greatest risk, as some studies have linked trihalomethanes to reproductive problems, including miscarriage.
Treatment Methods
There are several methods that people can use in their homes to reduce the trihalomethanes. Water well owners should always discuss these methods with a water treatment professional or water chemist before deciding to use one (water filters in the retail market are often sold with erroneous claims). Among the methods are:
- Filters
- Aeration or boiling
- Distillation
- Activated carbon — normally in the form of a filter
For a private groundwater supply, continuous treatment with chlorine or bromine is usually not recommended, and indeed prohibited in some US states. However, if it is necessary or desirable for some purpose, a carbon filter to remove the halogen and THM downstream is recommended. These may be whole-house or at point of use. If a well has been shock chlorinated, a downstream sediment and carbon filter is also recommended. Public water supplies with THM issues often switch to alternative chlorine methods, or disinfection choices. These may be considered for private water supplies as well.